Contaminated Eye Drop Recalls Endanger Washington Residents
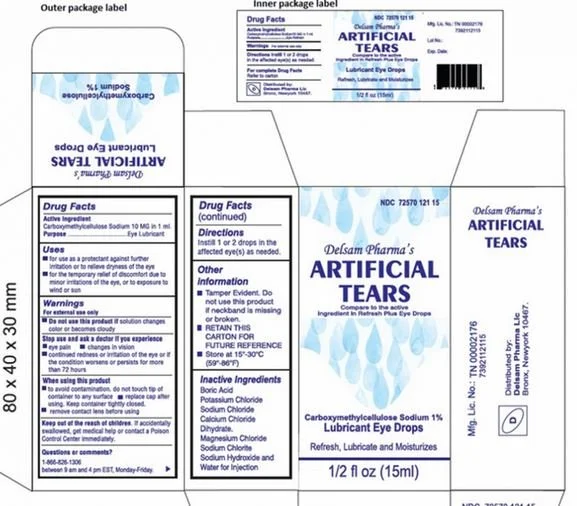
Ezricare and delsam pharma contaminated eye drops
On February 2, 2023 the FDA announced that Global Pharma Healthcare was voluntarily recalling eye drops that may be contaminated with drug resistant bacteria Pseudomonas aeruginosa. The eye drops were sold under brand names EzriCare, LLC and Delsam Pharma as Artificial Tears Lubricant Eye Drops. The full product labels can be viewed here.
The FDA cites “55 reports of adverse events including eye infections, permanent loss of vision, and a death with a bloodstream infection.” Here is an article explaining how healthcare officials “cracked the case” on these infections across multiple states.
Pharmedica and Apotex Issue New Eye Drop Recalls
Now, two more companies are recalling eye drop products. Pharmedica issued a recall of Purely Soothing eye drops over a risk of infection “due to non-sterility” “that could result in blindness.” Apotex has issued a recall of its Brimonidine Tartrate Ophthalmic Solution due to potential non-sterility. You can view the products at those FDA links.
Contaminated Eye Drop Injury Claims and Lawsuits
Under Washington law, manufacturers of defective products are strictly liable for injuries and damages caused by those defects. This means you do not have to prove the companies that made the product were negligent in manufacturing. You only have to prove that the contamination injured you.
Product sellers are also liable to people injured by the product when they sell the product under their own brand name. Even if the manufacturer is in another country, a product seller in the U.S. may be responsible for injuries caused by its products.
Products liability is one of my primary practice areas. If you or anyone you know has been impacted by these recalls, please feel free to contact me for a free consultation.
Update march 22, 2023
The CDC is now reporting two more deaths and additional cases of vision loss from EzriCare and Delsam Phama eye drops contaminated with Pseudomonas bacteria.
Update April 3, 2023
Scientists are now concerned that Pseudomonas aeruginosa linked to contaminated EzriCare and Delsam Pharma eye drops can be transmitted person-to-person. Originally reported by the New York Times, a free summary is accessible here.
Also, the FDA inspected Global Pharma Healthcare Pvt. Ltd., the eye drop manufacturer in India, and has now published its citation to the company. Recall notices have announced that Global Pharma violated Current Good Manufacturing Practices (CGMP). The citation details the FDA’s findings regarding sterility, cleaning processes, and company records.